Toul Meditech AB is proud to announce that Operio has been cleared by the FDA according to 510(k) and that the device now can be marketed in the US.
Operio has been cleared for the US market
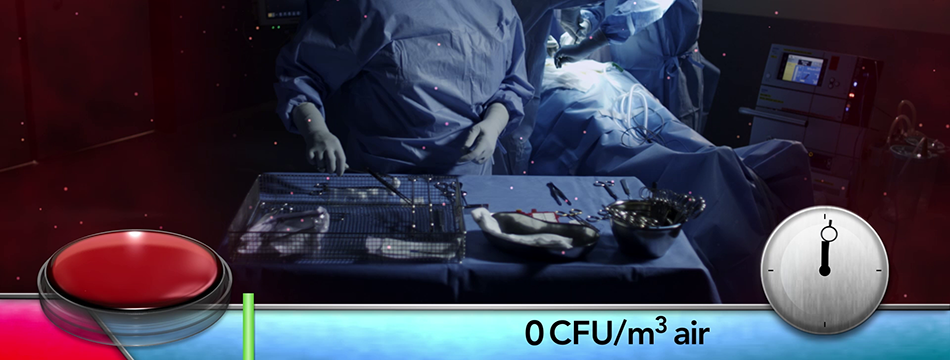
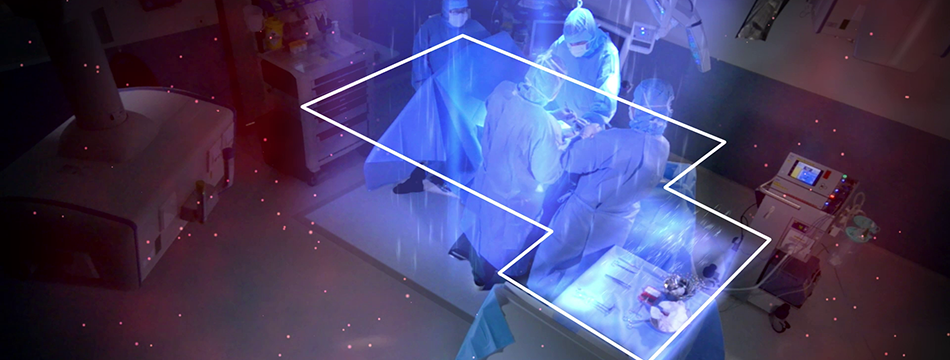
Operio is a portable device for use in a surgical operating room that produces a directed, non-turbulent flow of air to the surgical site during ophthalmic surgery and to the sterile instruments used during surgery.
Tomas Hansson, CEO Toul Meditech AB, 2016-07-25
Sterile air zone units for orthopaedic surgery at Drammen Hospital in Norway
Toul Meditech AB has recently sold and installed several sterile air zone units at 3 surgical operation rooms in the Drammen Hospital in Norway.
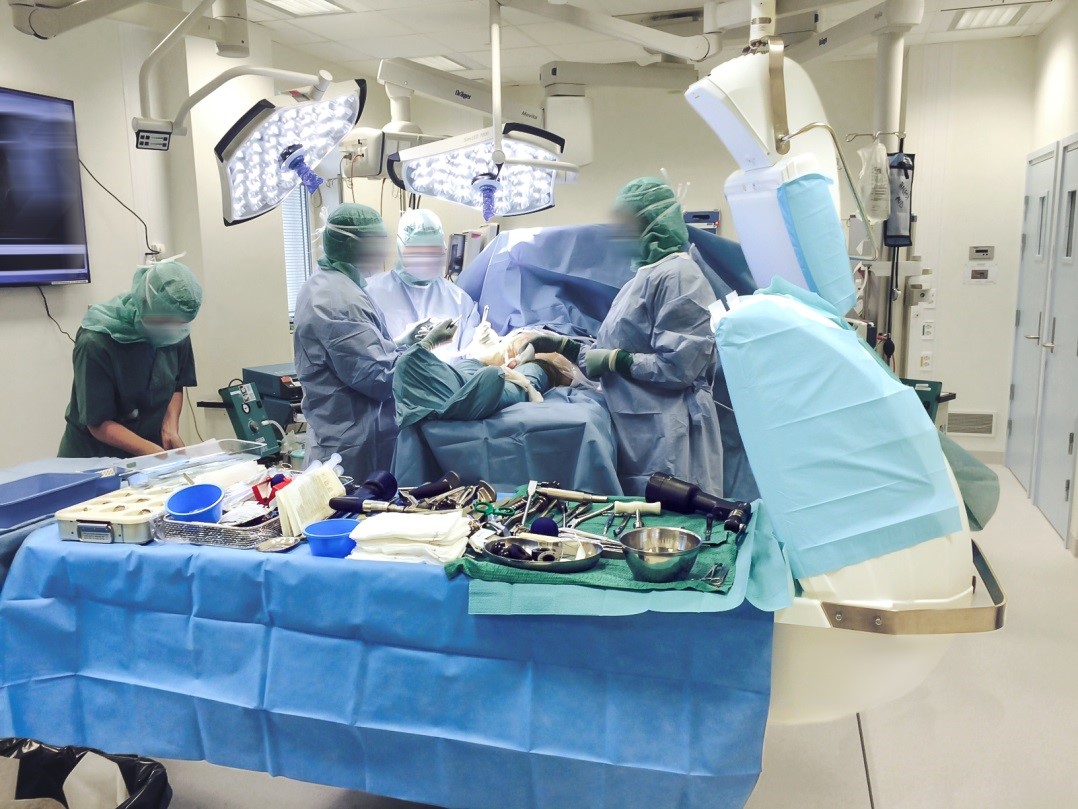
The devices are being used during all types of orthopaedic surgery.
The orthopaedic surgery department at Drammen has in recent times undergone a major refurbishment with a new ventilation system. In connection to this the Hospital decided to ensure that air cleanliness in the sterile zone could be maintained by using Toul equipment. The Operio Ceiling is used to protect the wound area and instruments during surgical procedures against air-borne bacteria and SteriStay is used to protect the instruments and implants against airborne bacteria.
Tomas Hansson, CEO Toul Meditech AB, 2015-09-11
TOUL Meditech AB joins SurgiCube International BV
We are happy to announce that TOUL Meditech AB has joined forces with SurgiCube International BV from The Netherlands, which has acquired TOUL from MGA Holding AB. SurgiCube International BV is also active in the field of infection prevention. The company has developed the SurgiCube®, a standalone surgical unit that provides a localized, optimally filtered, sterile surgical environment, offering a uni-directional laminar down flow technique, to carry out microsurgical procedures and minor surgeries. See www.surgicube.com.
The Toul units and shields have proven to be effective and successful across Sweden and in a number of other European countries, both in hospitals and in private clinics. The Toul units make an excellent addition to the SurgiCube® portfolio. Together, SurgiCube® and Toul can offer solutions for virtually any situation in which maximum patient safety is required.
Tomas Hansson, founder and director of TOUL Meditech AB:
“I am very enthusiastic about the new cooperation with SurgiCube International. This allows us to provide our solutions even faster to surgeons and patients across the world because our mutual purpose is: protection of patients against airborne surgical infections. Saving lives and saving money”.
Ger Vijfvinkel, director of SurgiCube International BV:
“Toul Meditech is a company with great potential and a valuable product portfolio. Tomas Hansson is an inspired entrepreneur and we look forward to working with him and his team. Both in the continuous development of new products and in joining the product portfolio’s and customer bases. There is a huge demand for solutions for infection prevention and this combination brings us in an even better position to provide these solutions.”
Spijkenisse / Västerås, 30 March 2015
SurgiCube International BV
Stevinweg 1
3208 KM Spijkenisse
+31 (0)181 760 600
Toul Meditech AB
Tungbytorpsgatan 31
721 37 Västerås
+31 (0)181 760 600 +46 (0)21 135 000
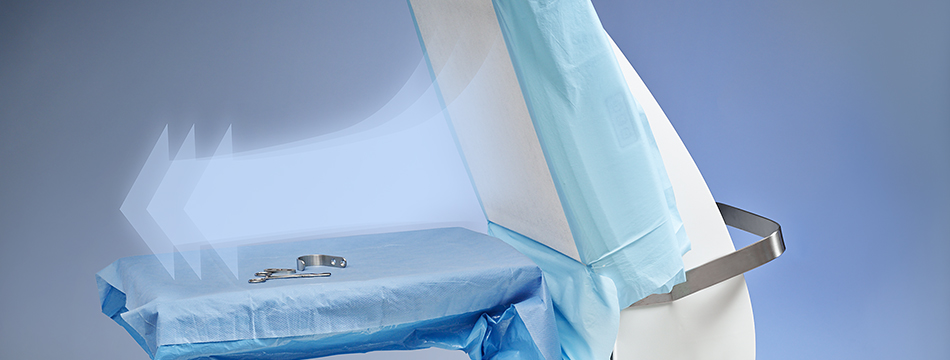
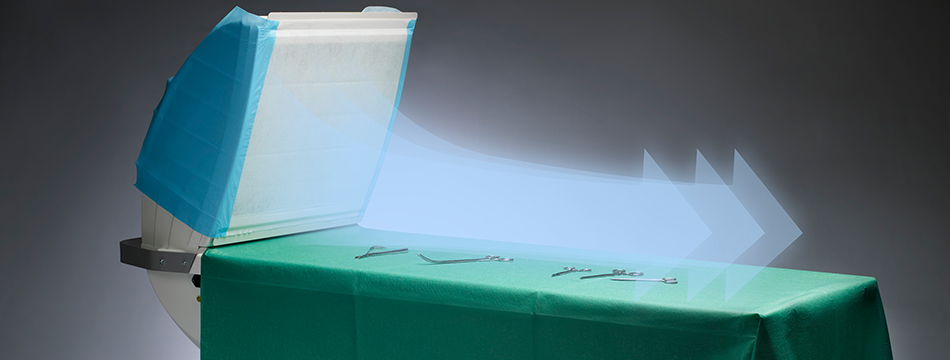
Toul Meditech (Västerås) has since 2003 successfully developed and marketed its patented and medical technology classified mobile systems for ultraclean laminar airflow. The airflow is distributed directed over the wound area and/or sterile instruments. The airflow constitutes a barrier which reduces the occurrence of bacteria carrying particles and by that the risk for post-op infections is reduced.
Ger Vijfvinkel, director of SurgiCube International BV, 2015-04-02
Video
SteriStay
Toul Meditech in 20 sec
Mobile airflow system for operating rooms