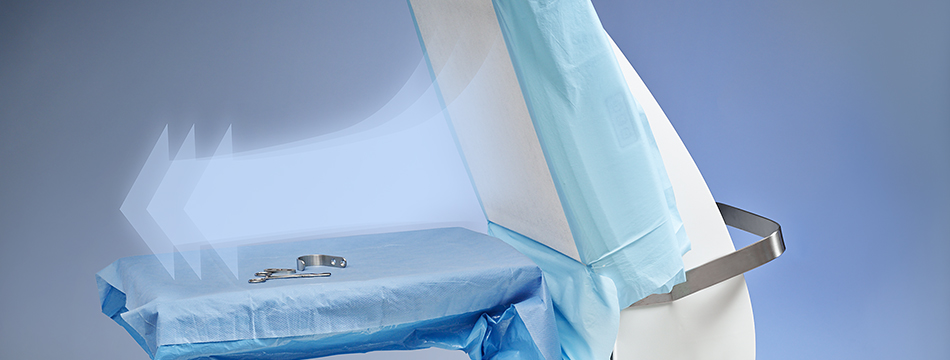
Mr. Madge first became aware of the Toul Meditech Operio® at the American Academy of Ophthalmology Annual Meeting 2019 in San Francisco, US.
“I had been looking for something like the Toul Meditech Operio® for quite some time, and I was quite surprised to see it in action,” he remarked. “It was very clear from the way that they were demonstrating it, using a particle counter to show how clean the air was, that it could easily be used to facilitate modern cataract surgery, as well as any other sort of intraocular procedure.”
While the Operio® answered the equipment needs that Mr. Madge had already had for some time, the timing of him discovering that it was available couldn’t have been better in the light of the ensuing pandemic a few months later.
Making Sight Saving Procedures Possible
When the Covid-19 pandemic started to impact the UK, many healthcare facilities were unable to continue their normal activities. The Toul Meditech Operio® provided the opportunity to keep going, creating a safe environment for cataract surgery.
“When Covid-19 hit in Hereford, we were unable to use our ophthalmic operating theater, because it was located next door to an intensive care unit,” explained Mr. Madge. “Even after the first Covid wave had come and gone, we still had a period of about four or five months, where due to a variety of local protocols, we couldn't get into the ophthalmic theater, and we had to choose between doing nothing, which of course, we knew was a very bad idea, or being innovative and act. We chose the latter. And the Toul Meditech Operio® allowed us to set up a safe, effective, and relatively high throughput cataract service within a standard NHS outpatient clean room - in other words, outside of an operating theatre. And that combined with some other innovative features of our pathway enabled us carrying out many hundreds of cataract operations in that sort of environment, with no infective complications, and a very high patient satisfaction.”
“It really got us ‘back on our feet’ again and restored sight to hundreds of people. Otherwise, we would have been paralyzed as a service,” he added. “I know, from subsequent discussions with lots of other ophthalmologists around the country, that some of them are still feeling that way – paralyzed, almost after a year after the pandemic started.”
Cleanest Possible Surgical Field
With a laminar air flow, Toul Meditech Operio® was developed especially for operation environments, in which demands on sterile and clean air are elevated.
The horizontal ultraclean laminar airflow is directed directly over the surgical site and instruments. This creates a barrier against particles that could harbor infective agents. With this surety, Mr. Madge uses the system for his work in private as well as NHS settings.
“Toul Meditech Operio® provides us with the cleanest possible surgical field better than standard operating theaters in many, many respects, and allowed us to resume private cataract surgery when the rest of the country was closed for business,” he remarked. “We're still using it, because we believe it is not only a great way forward from a financial perspective, but actually gives us the cleanest possible air for our surgery.”
Better Experience for Specialist and Patients
With inherent flexibility, the Toul Meditech Operio® is easy to maneuver, maintain and use. Specialists like Mr. Madge like the ergonomics of the system that benefit their own convenience and workflow, as well as patient comfort.
“It is a very, very flexible device. you can move it wherever you like, very simply and very quickly. It’s very easy to clean, and very easy to change the filters. The fact that you can move it around so quickly means that you can achieve superb efficiency in use,” said Mr. Madge. “With our own pathway in place within private practice, we were able to achieve well over two patients per hour, without any sensation of patients feeling rushed in anyway. It provides a very bespoke and positive patient experience and seems to go very well. I'm well aware that more than two operations per hour can be achieved with certain other parameters, but that's all we were striving for in this particular outpatient department.”
Fraction of the Costs
Another important element of the Toul Meditech Operio®’s appeal is the cost savings that can be achieved compared to conventional air conditioning systems.
“If you go down the traditional model of having a ceiling-mounted or even roof-mounted theater air conditioning system, which blows relatively sterile air through an entire room, your capital costs are going to be in the order with hundreds of thousands of pounds, along with tens of thousands of pounds on an annual basis for maintenance. That will achieve a room that is pretty sterile, but if there are then patients walking into that room in their shoes, their jeans, and their clothes, as happens normally in cataract surgery, it really begs the question: Why try to achieve a completely sterile room?” said Mr. Madge. “What the Toul Meditech Operio® does is create a sterile field for the areas that need to be sterile - the surgical field and the instruments. I honestly don't mind if my patients feet aren't bathed in sterile air, because it has no consequence to the outcome of the procedure in any way, shape or form. However, the Toul Meditech Operio® gives me ultraclean air, exactly where I need it, at a fraction of the cost.”
Extra Capacity for Post-Covid-19 Waiting Lists
While keeping operations running during the pandemic has helped treat hundreds of patients and minimize patient waiting lists, once the pandemic is under control, there will still be many more to treat. Mr. Madge is considering using the Toul Meditech Operio® to run an additional clinic for any extra capacity required.
“In terms of getting through the numbers of patients who might have been waiting for procedures, it would be lovely to open up an additional theater in a clean room in outpatients to help get through that,” he said. “If we have the space, that's what I will certainly choose to do. We would have the main operating theater for larger procedures, and we could continue with straightforward cataracts within an outpatient clean room environment. Within our private practice, the benefits of the Toul Meditech Operio® from a quality perspective are so great, that we plan to continue using it. I think the Toul Meditech Operio® is great and one of the best innovations there's been in the market.”
Mr. Simon Madge
Mr. Simon Madge is a Senior Consultant Eye Surgeon and Director of Hereford Vision Surgical Group, in Hereford, the UK, (www.HerefordVision.com). He is also Senior Consultant in Ophthalmic Surgery with an NHS practice based at Wye Valley NHS Trust, also in Hereford.
He studied medicine at Jesus College, Cambridge University, graduating with a Double First Class degree in Medical Sciences in 1994, before studying clinical medicine at Magdalen College, Oxford University. After initially training in general internal medicine and gaining Membership of the Royal College of Physicians, he then started specialist training in ophthalmology. After working in Bath and Nottingham, he completed his Higher Surgical Specialist Training on the Peninsular Rotation (Devon), gaining Fellowship of the Royal College of Ophthalmologists in 2007
In addition to his training in cataract / refractive surgery, he undertook two Fellowships in Eyelid, Lacrimal and Orbital Surgery: with Mr. V.T. Thaller at the Plymouth Royal Eye Infirmary; and with Professor Dinesh Selva, Dr Garry Davis and Dr James Muecke at the Royal Adelaide Hospital, Adelaide, Australia, widely regarded as one of the best training opportunities in this field in the world.
He is an examiner for the Royal College of Surgeons of Edinburgh and is Fellowship Director of the Hereford International Clinical Fellowship in Ophthalmology & Oculoplastics. He is the author of two successful ophthalmic textbooks and over 60 scientific ophthalmic papers.