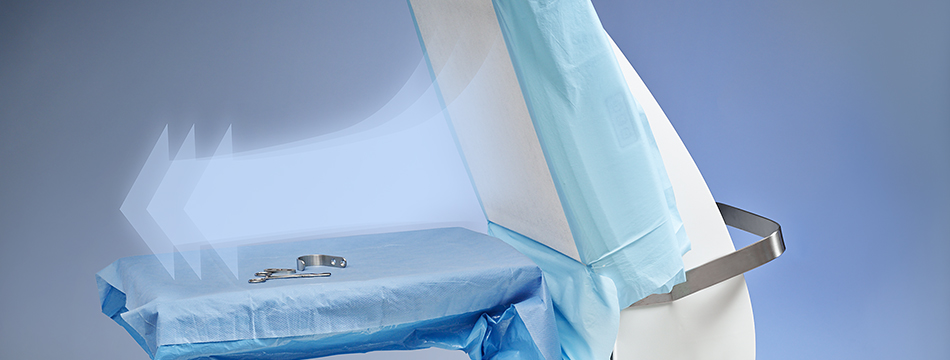
Toul Meditech recently got clearance from FDA to market the instrument table in the USA.
SteriStay has been cleared in the US
This opens up the market even further on the North American continent. SteriStay has been used successfully in Europe for several years as a standalone instrument table that protects the sterile instruments with HEPA filtered air. When both Operio and SteriStay is combined optimal effects can be reached in the fight against airborne contamination.
Tomas Hansson, CEO Toul Meditech, 2019-05-15
Published study on Operio in Neurosurgery
The study at Karolinska University Hospital involving Operio and SteriStay has recently been published in the Journal of Hospital Infection.
During the study a total of 233 samples were collected during 45 neurosurgical procedures. The use of MLAF units (Operio and SteriStay) significantly reduced the numbers of CFU in the surgical site area (P<0.001) and above the instrument table (P<0.001).
The name of the study is "Effect of mobile laminar airflow units on airborne bacterial contamination during neurosurgical procedures". To read about the result go to the following link: Journal of Hospital Infection.
Tomas Hansson, 2018-04-16
New distributor in USA
We are proud to introduce Aseptic Air Control as our new US distributor for Toul Meditech products in the USA.
Aseptic Air Control is a sister company to Aseptic Control Products Inc. who is a wholesale distributor specialized in aseptic products such as surface disinfectants, hand hygiene, instrument reprocessing, liquid medical waste, face protection and exam gloves.
With their broad experience and contacts in the infection prevention area they will be a perfect match for Toul Meditech products. The awareness around infection control and finding alternative solutions to antibiotics is increasing in the US, similar to the rest of the world. The US market is promising since both hospitals and insurance companies will gain in lowering hospital acquired infections. Not to forget the patient, who is the biggest winner.
We look forward to the cooperation and wish for great success. Toul Meditech was previously represented by Vitreq. They were recently acquired by BVI Beaver Visitec and will no longer carry our line of products.
To get in contact:
Aseptic Air Control
3831 Industrial Ave
Unit D
Rolling Meadows Illinois 60008
800-448-0131
847-342-1809 fax
847-342-1729
info@acpmedicalinc.com
Tomas Hansson, CEO, 2018-01-30
Operio has been cleared for additional indications by FDA
Operio has been cleared by the FDA for Neurosurgery and Orthopeadic surgery.
With the additional indications Toul Meditech sees an interesting market for neurosurgery and especially orthopeadic when it comes to providing clean air over the surgical site.
Tomas Hansson, CEO, 2017-12-22
Operio has been cleared for the US market
Toul Meditech AB is proud to announce that Operio has been cleared by the FDA according to 510(k) and that the device now can be marketed in the US.
Operio is a portable device for use in a surgical operating room that produces a directed, non-turbulent flow of air to the surgical site during ophthalmic surgery and to the sterile instruments used during surgery.
Tomas Hansson, CEO Toul Meditech AB, 2016-07-25