“We are pleased to welcome SurgiCube and Toul Meditech to the MedCap group and look forward to supporting the company’s continued growth journey, where the company can provide cost effective solutions to raise capacity for surgeries at hospitals and enable flexible high-quality solutions for smaller office-based surgery clinics.”, says Anders Dahlberg, CEO MedCap AB.
MedCap acquires Toul Meditech
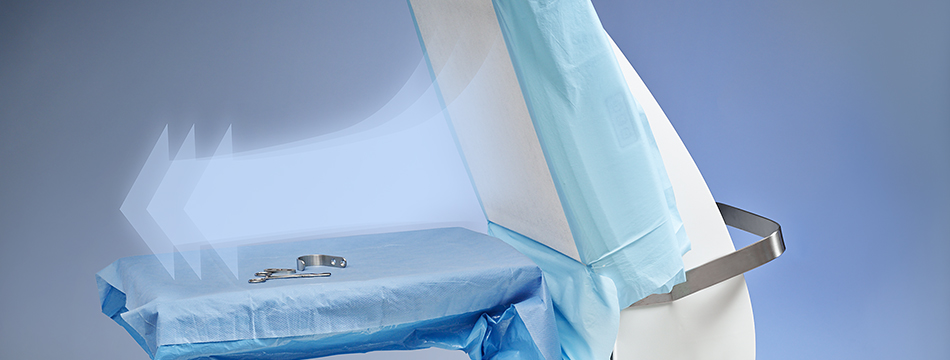
Toul Meditech AB is part of the he SurgiCube group and provide products for ultra clean air around the operating surface and is a high quality and cost-effective solution for both hospitals and smaller clinics. The solutions are used in both ophthalmic and orthopedic surgery; and are mainly sold throughout Europe. Over the past 10+ years the business has established a strong offering, with an installed base and recurring revenue, in a niche that is expected to continue to grow.
About MedCap
MedCap builds successful life sciences companies to improve people's lives. MedCap unites the strengths of a larger the company with the smaller companies' entrepreneurial power, agility, and business acumen. MedCap is publicly listed on NASDAQ Stockholm OMX, with the symbol MCAP. More information is available on the company web site www.medcap.se.
Tomas Hansson, 2023-07-25
Positive results from a study in Herefordshire, UK
Initial experiences of cataract & lens surgery in 1269 patients in outpatient clean rooms using a portable laminar air flow device.
BACKGROUND: In 2020, routine cataract surgery was halted in most countries due to the COVID-19 pandemic in order to reduce transmission. With a consequent lack of theatre space, we developed a safe cataract pathway in outpatient department clean rooms to minimize patient exposure and time spent in hospital using a sterile laminar air flow device. We describe our initial experiences of restarting elective cataract surgery in the UK outpatient setting, outside of the operating theatre environment.
METHODS: This was a prospective consecutive study of our clinical practice. A sterile air zone unit, the Toul Meditech Operio Mobile device, was used to create a sterile surgical site in three separate outpatient clean rooms from May 2020 to December 2021 in different geographical locations within Herefordshire, UK. Observations of the time spent in the department and a formal patient satisfaction survey were carried out for the initial 100 patients. All patients were followed up to assess development of postoperative complications.
RESULTS: 1269 patients were included in the study. No patients sustained post-operative infection (n = 0/1269, 0%). For the initial 100 patients, the average time spent within the department was 74.3 min (unilateral cases, range 45–115 min) and 93.1 min (bilateral, 55–135 min). Patient satisfaction was high.
CONCLUSION: Initial results demonstrate a safe, efficient and effective cataract surgery pathway with high patient satisfaction by converting outpatient clean rooms into ophthalmic operating theatres using the Toul Meditech Operio Mobile.
Read more at https://www.nature.com/articles/s41433-022-02317-7
Tomas Hansson, CEO, 2022-11-29
Study focuses on the space surrounding the sterile field
Can Airborne Particulates Contaminate Surgical Wounds?
The science says they can as some facilities respond with enhanced technology to stop them from harming patients.

Efforts to protect patients from avoidable harm should include a focus on the space surrounding the sterile field, suggests a recent study.
Publishing in the journal Surgery, the researchers say that although the accepted standard on the ventilation of ORs requires a minimum of 20 air changes per hour with a minimum of four outdoor air exchanges, externally sourced air can still present microbial and chemical contaminates to the OR.
Read the article in Outpatient Surgery Magazine
https://www.aorn.org/outpatient-surgery/articles/enews-briefs/nov-17-2022
Tomas Hansson, CEO, 2022-11-29
Positive study results for SteriStay and Operio
The study investigated instrument tables (Operio and SteriStay) equipped with local unidirectional airflow units reduce bacterial contamination during orthopedic implant surgery in an operating room with a displacement ventilation system.
The study was performed at Sahlgrenska/Mölndal Hospital in Sweden.
188 air and 124 instrument samples were collected during 48 orthopedic implant procedures. Analysis showed that local unidirectional airflow above the surgical instruments significantly reduced the bacterial count in the air above assistant table (P<0.001) and instrument table (P=0.002), as well as on the instrument dummies from the assistant table (P=0.001).
Read more about the study at: https://www.sciencedirect.com/science/article/pii/S2590088922000233?via%3Dihub
Tomas Hansson, CEO, 2022-06-13
Italian study published in European Journal of Ophthalmology
Intravitreal Injections during the COVID-19 Outbreak in Northern Italy. An Innovative Approach for a High Quality and Safe Treatment.
The 2020 COVID-19 outbreak caused a dramatic modification in outpatient care. Consequently, non-urgent surgical activities, like IVIs, were subjected to a drastic reduction.
An observational study was conducted which investigated the outcomes of IVIs performed in an ophthalmologist’s office using a mobile laminar flow unit, the Operio mobile (Toul Meditech, Operio®) versus an operating room setting.
This study was performed between March and July 2020. Five centers located in the North of Italy and one center located on one of the major islands participated in the project.
Use of the Operio mobile allowed the safety performance of 3838 IVIs during COVID-19 and significantly reduced the waiting time of the first visit. This results in a faster intervention without affecting the technical IVI procedure that remained unchanged comparing the two settings. Specifically, they observed a 26% reduction in operation costs for each IVI performed in the office, which can be translated to a higher impact when considering the total number of IVIs performed over one year.
The conclusion was that the use of the Operio mobile in an ophthalmologist’s office provides flexibility to perform IVIs, assuring patient safety, reducing healthcare personnel employment times, and the waiting lists for the patients, increasing the number of surgeries and improving the cost-effectiveness of the procedure.
To read the full article go to:
https://journals.sagepub.com/eprint/HKEZTE4ZDVKUJC3HK5B5/full
Tomas Hansson, CEO, 2022-02-10